Trusted by Industry Leaders, Globally
95% Repeat Business. 100% Happy Clients
What we do?
Formulations
The scientific thrill of discovering and refining solid oral formulations is so intense. This requires interaction of the various departments such as formulation development, analytical method development, analytical method validation and so on. In the process, collision of formulation scientists, analytical chemists and regulatory professionals is natural. But it is a constructive collision; something that results in a beautiful creation. Remember the Big Bang that made life possible on planet earth?

Manufacturing
The harmony of competent people, quality systems, state-of-the-art facilities, cutting-edge machines, and clean environment can produce magic, consistently. It is what makes us a deft manufacturer of tablets, capsules, dry syrup, hand sanitizers and many more. Be it the complex “double coated bi-layer tablets” or the simple “fill & seal” sachets, Good Manufacturing Practices (GMP) never ceases to amaze us. Machines can behave in so many different ways just by tweaking a few process parameters.

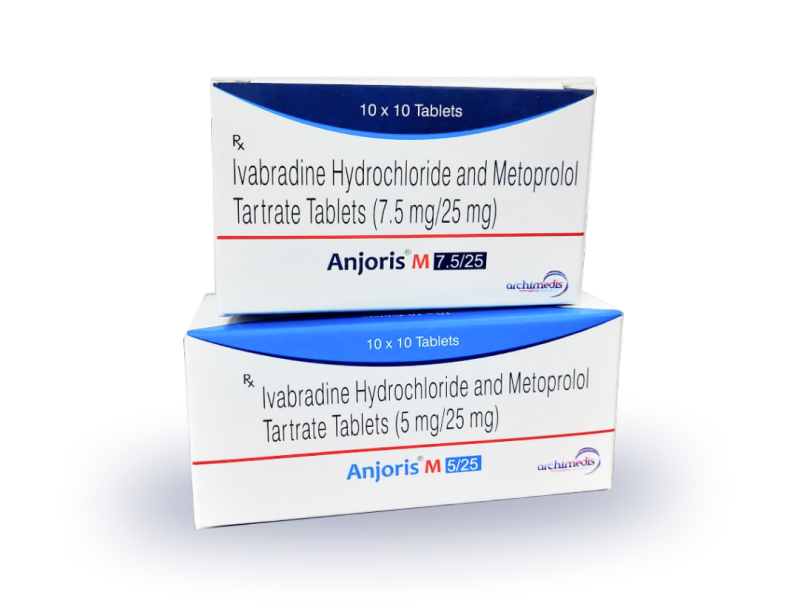
Products
Across therapeutic areas, our Tablets, Capsules & Dry Syrup products are packed in HDPE Bottles, Alu-Alu, Blister, Sachet and Strip formats. We specialize in non-beta lactam based solid oral products with years of experience in making prescription drugs and food supplements. Additionally, our online store has other OTC & Rx products sourced from curated GMP partners.
Digital
Digital by Default; Manual by Exception” has become the norm in the Life Sciences industry. From DevOps to Industry 4.0, our digital business unit offers digital product development, product support, ERP solutions, smart dispensing, environmental monitoring and custom implementation services. We also specialize as a pharmaceutical digital solutions provider in IT Quality & Compliance, including but not limited to GxP compliant SDLC design, Computerized System Validation (CSV), Software Quality Assurance (SQA), Software Test Automation, and Part 11 / Annex 11 Controls.

200+
Employees

50+
Clients
450+
Products
Why Archimedis?
Our single-minded focus on superior quality leading to client success.
Robust Processes
Capable People
Latest Technology
Consistent Execution
Don't just take our word for it, hear what our clients have to say...
Clients Speak
Get to Know Us
We aspire to further enhance life on earth by formulating scientific & digital innovations. We passionately architect medicine and digital systems so that planet earth continues to thrive.
Board of Directors

Palani Duraisamy
Chariman and Managing Director

Dr. Suresh Kumar Palani
Chief Mentor & Non-Executive Director

Ramesh Kumar Palani
Executive Director

Duraisamy Rajan Palani
Executive Director
Our Latest Thinking

The Art of Drug Design
The inventive process of finding new medications based om the knowledge of a biological target aka drug design, can be complex task.We describe the intricacies of this elaborate process.
March 13, 2021
Three Strategies to Improve Structural Integrity of Bi-layer Tablets
Bi-layer tablets are not only difficult to compress. They also carry an inherent risk of splitting during coating. Our experience uncovers five factors that can help reduce the risk.
February 11, 2021

The Open Source Myopic in Life Sciences
Digital has influenced nearly every sector in the modern business world and life sciences is no different. Yet, given the complexity of the life sciences industry the options available seem lacking.
December 25, 2020
Contact us
Reach Us
We would love to hear from you!
+91 44 4717 1111
care@archimedis.net